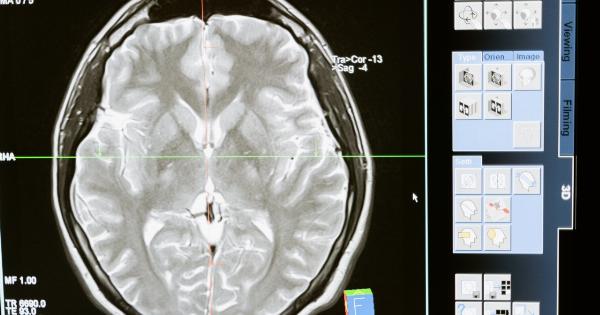
Alzheimer’s disease is a debilitating condition that affects millions of people worldwide, with the number of cases expected to rise dramatically in the coming years.
While there is not yet a cure for the disease, recent developments in medication have given hope to many individuals and their families.
What is Alzheimer’s Disease?
Alzheimer’s disease is a progressive neurological disorder that initially presents as mild memory loss, but eventually leads to severe cognitive decline and significant impairment in daily functioning.
The disease affects the brain’s ability to create and maintain connections between nerve cells, leading to a loss of brain tissue and the formation of abnormal protein deposits known as amyloid plaques.
While the exact cause of Alzheimer’s disease is not yet known, scientists believe that a combination of genetic, environmental, and lifestyle factors contribute to the development of the disease.
Risk factors for the disease include age, family history, certain medical conditions, and exposure to toxins and other environmental factors.
The Current State of Alzheimer’s Treatment
There is currently no cure for Alzheimer’s disease, and available treatments are limited in their ability to slow or prevent the progression of the disease.
Medications like cholinesterase inhibitors and memantine can help temporarily alleviate symptoms like memory loss and confusion, but they do not stop the underlying neurodegenerative processes that cause the disease. Additionally, these medications often come with side effects that can be difficult for patients to tolerate.
Recent Developments in Alzheimer’s Medication
Researchers have been working for decades to develop medications that can slow or stop the progression of Alzheimer’s disease.
One promising approach is the use of drugs that target the formation of amyloid plaques, which are thought to play a significant role in the development of the disease.
For many years, scientists struggled to develop effective drugs in this category, with many potential treatments failing in clinical trials. However, in June 2021, the U.S.
Food and Drug Administration (FDA) approved a new medication that shows significant promise in slowing the progression of Alzheimer’s disease.
The Approval of Aduhelm
The newly-approved medication, called Aduhelm (generic name: aducanumab), is a monoclonal antibody that targets amyloid plaques in the brain.
The drug is administered through intravenous infusion, and is given in monthly doses over an extended period of time.
The approval of Aduhelm is a significant development in the fight against Alzheimer’s disease, as it is the first medication in over 18 years to receive approval from the FDA for the treatment of the disease.
The drug was granted accelerated approval based on data from two clinical trials that showed a reduction in amyloid plaques in the brain of patients who received the drug, as well as a slowing of cognitive decline.
The Controversy Surrounding Aduhelm
While the approval of Aduhelm has given hope to many individuals and families affected by Alzheimer’s disease, it has also been met with significant controversy.
Some experts have criticized the FDA’s decision to grant accelerated approval, citing concerns about the drug’s efficacy and safety.
One particularly contentious issue is the lack of long-term data on the drug’s effects.
The clinical trials for Aduhelm were relatively short, lasting only 18 months, and there are concerns about the potential risks associated with long-term use of the drug. Additionally, the drug is expensive, with estimated costs of over $50,000 per year, which could make it difficult for many patients to access.
The Future of Alzheimer’s Treatment
Despite the controversy surrounding Aduhelm, the approval of this medication represents a significant step forward in the fight against Alzheimer’s disease.
It is expected that additional drugs targeting amyloid plaques will be developed in the coming years, along with other treatments that target different aspects of the disease.
In addition to pharmacological treatments, researchers are also exploring other approaches to treating Alzheimer’s disease, such as lifestyle interventions and non-invasive brain stimulation techniques.
These interventions aim to improve brain health and function, and may be effective in slowing or preventing the progression of the disease.
Conclusion
The approval of Aduhelm is a positive development in the fight against Alzheimer’s disease, but there is still much work to be done to improve treatment options for this devastating condition.
Scientists are continuing to explore new approaches to treatment, and it is hoped that in the coming years, we will see significant progress in the fight against Alzheimer’s disease.