A groundbreaking new blood test has been developed that is capable of identifying brain tumor cells in a non-invasive manner.
This revolutionary technique has the potential to transform the way brain tumors are diagnosed and monitored, allowing for earlier detection and personalized treatment plans.
The current challenges in diagnosing brain tumors
Diagnosing brain tumors can be incredibly challenging, requiring invasive procedures such as brain biopsies or surgical interventions. These methods can be risky, time-consuming, and often provide limited information about the tumor.
Additionally, the symptoms of brain tumors can be vague and easily confused with other conditions, leading to delays in diagnosis and treatment.
Furthermore, brain tumors are highly heterogeneous, meaning that they can vary greatly in terms of their genetic makeup and response to therapies.
This makes it critical to accurately identify the specific characteristics of a tumor in order to develop an effective treatment plan.
A new era in brain tumor diagnosis
The newly developed blood test aims to revolutionize the field of brain tumor diagnosis by offering a non-invasive and efficient method of identifying tumor cells.
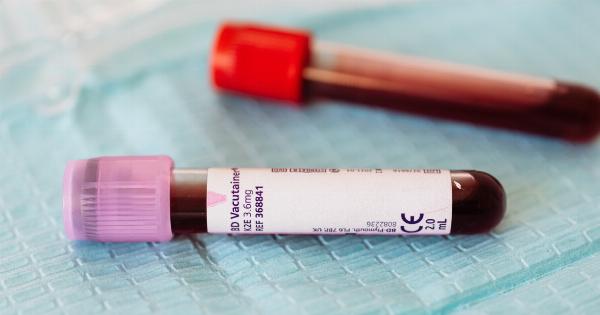
This test, known as liquid biopsy, works by detecting fragments of tumor DNA that are shed into the bloodstream.
Traditionally, liquid biopsies have been primarily used for the detection of genetic mutations in cancer cells. However, this innovative blood test takes it a step further by specifically targeting brain tumor cells.
By analyzing the genetic material within the blood sample, scientists can accurately identify and characterize the tumor cells without the need for surgical procedures.
How does the blood test work?
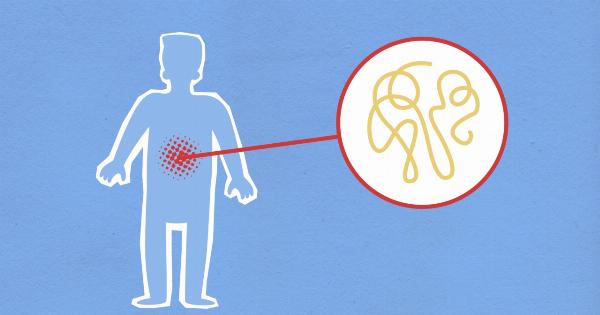
The blood test relies on the fact that tumor cells release small fragments of their DNA into the bloodstream, known as circulating tumor DNA (ctDNA).
These fragments can be isolated from a blood sample and analyzed for genetic abnormalities that are indicative of a brain tumor.
Researchers have developed highly sensitive techniques to detect and analyze these ctDNA fragments.
By using specific markers or genetic signatures that are unique to brain tumors, they can differentiate between tumor-derived DNA and normal DNA present in the blood sample.
Additionally, advancements in next-generation sequencing technologies have made it possible to sequence the entire genome or specific regions of the tumor DNA.
This comprehensive analysis provides valuable insights into the genetic makeup of the tumor, including potential therapeutic targets and drug resistance mutations.
Advantages of the blood test
The revolutionary blood test offers several advantages over traditional brain tumor diagnostic methods:.
1. Non-invasive
The blood test is completely non-invasive, eliminating the need for surgical procedures or biopsies. This not only reduces the risk and discomfort for patients but also allows for frequent monitoring without added stress.
2. Early detection
The blood test has the potential to detect brain tumors at an earlier stage, even before symptoms manifest. Early detection is crucial as it increases the chances of successful treatment and improved patient outcomes.
3. Personalized treatment
By analyzing the genetic makeup of tumor cells, the blood test enables the development of personalized treatment plans. This allows for targeted therapies that have a higher likelihood of success and reduces the risk of unnecessary treatments.
4. Monitoring treatment response
The blood test can be used to monitor the effectiveness of treatment over time. By analyzing changes in ctDNA levels or specific genetic alterations, doctors can assess whether the treatment is working or if modifications are necessary.
The future of brain tumor diagnosis and treatment
The development of this revolutionary blood test marks a significant milestone in the field of brain tumor diagnosis. It offers a promising avenue for faster, more accurate, and individualized detection of brain tumors.
This advancement opens up possibilities for better treatment outcomes, improved patient experiences, and enhanced overall survival rates.
As further research and validation are undertaken, it is anticipated that this blood test will become an essential tool in the diagnosis, monitoring, and treatment of brain tumors.