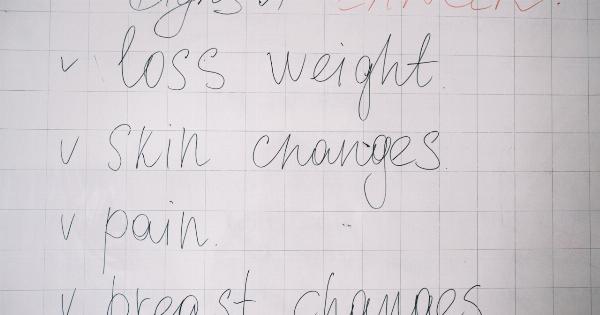Uterine cancer, also known as endometrial cancer, is one of the most common gynecological malignancies worldwide. It primarily affects the lining of the uterus called the endometrium.
Early detection and diagnosis of uterine cancer are crucial for increasing the chances of successful treatment and improving patient outcomes. In recent years, the Cobas A Test has emerged as a promising tool for early detection and screening of uterine cancer.
Understanding Uterine Cancer
Uterine cancer occurs when abnormal cells in the endometrium grow and divide uncontrollably. This abnormal cell growth can lead to the formation of a tumor, which can invade nearby tissues and potentially spread to other parts of the body.
The exact cause of uterine cancer is unknown, but certain risk factors such as obesity, high blood pressure, diabetes, hormonal imbalances, and a family history of uterine cancer can increase a person’s likelihood of developing the disease.
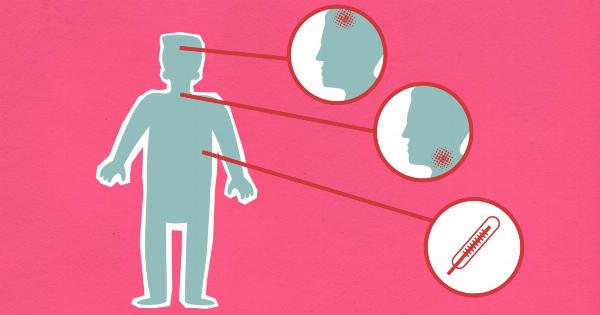
Signs and Symptoms of Uterine Cancer
One of the challenges in diagnosing uterine cancer is that early-stage symptoms can often be subtle or easily confused with other common conditions.
However, being aware of these signs and symptoms can prompt individuals to seek medical attention and potentially catch the cancer at an early stage. Common symptoms of uterine cancer include:.
- Abnormal vaginal bleeding, such as spotting or bleeding between periods
- Heavy or prolonged menstrual periods
- Pelvic pain or discomfort
- Unexplained weight loss
- Feeling a mass or lump in the pelvic area
The Role of Cobas A Test in Early Detection
Cobas A Test is a revolutionary diagnostic test that has shown promise in the early detection of uterine cancer. It is a non-invasive test that analyzes specific biomarkers associated with uterine cancer in a patient’s blood sample.
By detecting these biomarkers, the Cobas A Test can identify the presence of uterine cancer at an early stage when it is more treatable.
Advantages of Cobas A Test
There are several advantages to using the Cobas A Test for early detection of uterine cancer:.
- Non-invasive: Unlike traditional diagnostic methods such as biopsies, the Cobas A Test does not require any invasive procedures. It only requires a simple blood sample.
- Early Detection: The Cobas A Test can identify uterine cancer at an earlier stage compared to other diagnostic methods, allowing for prompt treatment initiation.
- Accuracy: Studies have shown that the Cobas A Test has a high sensitivity and specificity for detecting uterine cancer, minimizing the risk of false-positive or false-negative results.
- Convenience: The ease of collecting a blood sample makes the Cobas A Test convenient for patients and healthcare providers alike.
Implementing the Cobas A Test
As with any diagnostic test, the successful implementation of the Cobas A Test for early detection of uterine cancer requires careful consideration and coordination between healthcare providers and laboratories.
Here are some key steps in implementing the test:.
- Educating Healthcare Providers: Ensuring that healthcare providers are aware of the Cobas A Test and its benefits is crucial. Training sessions and educational materials can help disseminate information about the test.
- Establishing Laboratory Protocols: Laboratories need to establish standardized protocols for processing and analyzing blood samples for the Cobas A Test to ensure accurate and reliable results.
- Integration into Screening Programs: The Cobas A Test can be integrated into existing uterine cancer screening programs to enhance early detection efforts and improve overall screening outcomes.
Conclusion
Early detection of uterine cancer plays a pivotal role in improving patient outcomes and increasing the chances of successful treatment.
With its non-invasive nature and high accuracy, the Cobas A Test has emerged as a promising tool for early detection and screening of uterine cancer. By implementing this test and raising awareness among healthcare providers and patients, we can take a significant step forward in the fight against uterine cancer.